

Exercises with Mass, Binding Energy and the Liquid Drop Model
1:
#Mass excess is given by ΔM(Z,A) = M(Z,A)-A. The masses can therefore be found with M(Z,A) =ΔM (Z,A ) + A by using 1 u = 931.5 MeV we get:
- M(n) = 1.008665 u
- M(1H) = 1.007825 u
- M(4He) = 4.002603 u
- M(56Fe) = 55.934938 u
- M(142Ce) = 141.909197 u
- M(238U) = 238.050788 u
- The most stable nuclei is 56Fe. It has the highest binding energy compared to the number of nucleons.
- 1.00 kg 2H is 496.5 moles by fusion 248.2 mole 4He is created which is 993.6 g. The difference in mass is equal to the energy 1000 -993.6 g = 6.36 g. The energy is then 5.7•1014J, 3.56•1027MeV or 1.59108kWh.
- 1.00 kg 233U fission to 92Rb, 138Cs and three neutrons. The mass difference is 0.785 g. Which gives 7.1•1013 J, 4.40 •1026 MeV or 1.96•107 kWh.
- The energy from the fission is distributed in different ways: most of it goes to the kinetic energy of for the fission products. Other parts go to the kinetic energy of the neutrinos and the neutrons, and some of it goes to “prompt” gamma-rays and beta/gamma rays from the fission products.
2:
Binding energy B per nucleon:
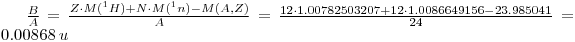
It is 0.00868 u per nucleon and 8.26 MeV per nucleon
3:
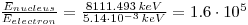
1.6•105 times larger binding energy for the nucleus.
4:
- mn = 1.67•10-27kg
- me = 9.11•10-31kg
- mu = 1.66•10-27kg
5:
- 8.55 MeV/nucleon.
- 8.79 MeV/nucleon.
- 7.86 MeV/nucleon.
6:
The energy
M(235U) - (M(131Xe + M(101Ru) + 3M(n)) =
(ΔM(235U) + 235) - (Δ(131Xe) + 131) - (ΔM(101Ru) + 101) - 3(ΔM(m) + 1)
= ΔM(235U) - ΔM(131Xe) - ΔM(101Ru) - 3ΔM(n)
=40.916 + 88.421 + 87.952 - 3• 8.071 = 193.08 MeV
7:
Energy usage per 10 km:
1 L = 700 g. Mm(C8H18) = 114 g/mole, this means that 700 g is 6.14 mol.
6.14 mole•5500 kJ/mole = 33771.93 kJ, with a fuel efficiency of 18% the usage per 10 km is 6078.94 kJ/(10 km).
1 g 235U=4.25•10-3 mole = 2.56•1021 atoms.
If all of the atoms fission and assuming 100% efficiency this will give 5.12•1029 eV = 8.22•107kJ Which will last for 135190 km.
8:
ΔG = ΔGproduct - ΔGreactant= - 237 kJ/mol. Which means that for each H2O molecule it is generated 237kJ/NA = 2.456•10-6 MeV. Comparing to 0.0303 u = 23.2 MeV released by fusion to helium. We see that the nuclear reactions functions on a scale 106 times bigger than chemical.
9:
Fusion of 2H gives 5.75•1011 J/gram approximately ten times more energy then uranium which gives 8.2•1010 J/gram.
10:
In this problem it is useful to keep in mind the binding energy equation:
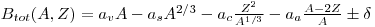
Most isobars (A= constant, Z varies) will have binding energy when plotted as a function of Z creating a parable. The last part of the equation in the formula for binding energy delta expresses the energy gained when the proton and/or the neutron are in a couple. A nucleus with an even amount of both will have a positive δ, higher binding energy, an unpaired nucleus either proton or neutron will have a δ of zero, a nucleus with a unpaired neutron and proton will have a negative δ. Therefore for odd-nuclei the binding energy is independent of which nucleon is unpaired. The binding energy as a function of z will have a minimum value for a certain stable nucleus. For even nuclei with a given A value they will have an even-even, or an odd-odd configuration when Z varies. This will create two curves where the odd-odd nuclei will have a lower binding energy than the even-even nuclei. Odd-odd nuclei will therefore have more possibilities when decaying to a level with higher binding energy, either with a β- decay or a β+ decay. Both of these mechanisms will lead to a more stable nucleus.
11:
Answered above